Below, you'll find answers to common questions about bacterial toxins. This guide will help you understand bacterial toxins, how they impact your gut health, and how the Tiny Health Gut Test can identify them. To access this feature, log in to your account to explore your existing gut health report or purchase a Gut Health Test.
What are bacterial toxins?
Bacterial toxins are like tiny weapons that cause illness. They can target different parts of our body and mess up normal functions. For example, some toxins attack our cells, causing cell death. Others impact how the systems in our bodies talk to each other, leading to inflammation and other health issues [1]. Our gut microbiome plays a role in this, too. Most gut bacteria are helpful. But some can make toxins that can cause digestive issues and affect our health.
Does the Tiny Health gut test detect actual bacterial toxins in the gut?
While the Tiny Health Gut Test doesn’t show the toxins made by bacteria in a direct way, it identifies genes in the bacteria that have these toxins that we call “bacterial toxin genes”. Each bacterium carries a unique set of genes, including some that make toxins. By looking at the microbial DNA in your gut, the test can find out if your gut bacteria might produce harmful toxins.
Where do I find bacterial toxins in my report?
In the ‘Disruptive microbes’ category of your Gut Health Test report, you’ll find the section Opportunistic Pathogens. In this list, some species may highlight toxins detected. Click any of them to view the details of the toxins.
My sample is positive for bacterial toxin genes. What does this mean?
Certain bacteria in your gut can potentially produce toxins. But it's important to know that if your sample is positive, it doesn’t mean toxins are active or causing harm now. Their presence shows that these bacteria could produce toxins under certain conditions. Your diet, lifestyle, immune system, and gut health help influence if these bacteria become active, make toxins, and cause illness (Figure 1).
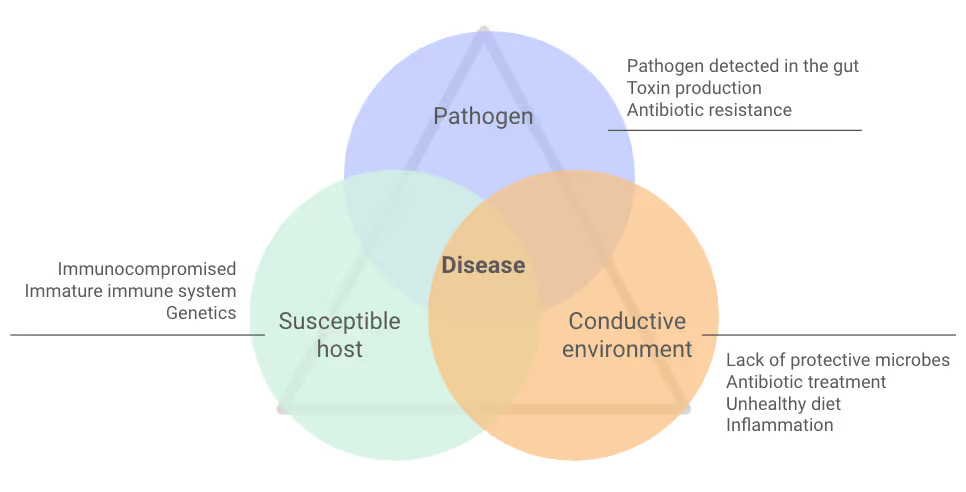
What does it mean if my sample doesn't have all the genes for a toxin?
Having only some genes for a toxin means the bacterium doesn't have all the instructions needed for the toxin to work. Bacterial toxins often have many parts that work together to harm the body. If your results show only some of the toxin genes, it means the bacteria in your gut might be able to make part of the toxin.
In other words, these bacteria might not make you as ill as ones with all the toxin genes.
My results show an opportunistic pathogen, but bacterial toxin genes were not detected. What does that mean?
Some strains of the same bacterial species can have different toxin genes. This means that while specific strains can make toxins, others might not have these genes at all or have different versions of them.
Sometimes, toxin genes can live on DNA that bacteria can pass around or lose over time.
Results are often different when opportunistic pathogens don’t have toxin genes. If you get an infection, it could be less severe than one with toxin genes. Without toxin genes, pathogens can't do as much harm.
It's also possible that the toxin genes are there, but in such small amounts that they aren’t detected.
Can having toxin genes in my gut predict health outcomes?
Sometimes. Having toxin genes can increase the risk for certain conditions. But it doesn’t mean you'll get them.
For example, if you have Clostridioides difficile (C. diff) in your gut, you might get diarrhea after taking antibiotics. If you test positive for C. diff’s toxin A and B gene, it means the C. diff can cause severe antibiotic-related diarrhea. If this happens, supporting your gut during and after antibiotics is important.
In short, having a bacterial toxin gene doesn't always mean you will get a specific health problem. But it shows that some opportunistic pathogens in your gut might be able to cause disease.
What can I do to reduce the levels of bacterial toxin genes in my gut microbiome?
To lower the levels of bacterial toxin genes in your gut, you need to target specific opportunistic pathogens that carry them.
When you balance your gut bacteria, it helps to reduce the harmful germs that carry these toxin genes. If your levels are high, your Action Plan will likely recommend that you:
- Eat a balanced diet rich in fiber
- Avoid processed foods and sugars
- Take a targeted probiotic supplement
What bacterial toxin genes do Tiny Health gut tests identify?
Below is the list of toxin genes that may show up in the Tiny Health Gut Test.
Staphylococcus
Exfoliative toxin type A attacks desmoglein-1, a key protein that helps skin cells stick together. When the cells disconnect, blisters occur [2]. The toxin's role in the gut is unknown because it targets skin cells. But, if the gut has Staphylococcus, it might increase the risk of skin infections [3].
Staphylococcal enterotoxins cause food poisoning. Staphylococcal enterotoxins cause a strong inflammatory response and help the infection spread [4]. After eating contaminated food, severe diarrhea, nausea, and stomach cramps may occur [5]. They can also cause toxic shock syndrome. These toxins are often found in children’s guts [6], [7].
Toxic shock syndrome toxin 1 makes immune cells release substances that cause inflammation, fever, and shock [8].
Leukocidins are toxins that make holes in the surface of immune cells and red blood cells. They disrupt cell balance and lead to inflammatory cell death [9].
Superantigen-like protein 1, Superantigen-like protein 5/11, Superantigen-like protein 7 are harmful Staphylococcus aureus toxins. They change how immune cells work and allow the bacteria to survive and stay in the body [10], [11].
Streptococcus
C3 family ADP-ribosyltransferase disrupts the inside of immune cells and makes the bacteria more harmful [12], [13], [14].
cAMP factor forms bubbles inside macrophages, making it harder for them to destroy pathogens or foreign particles [15], [16]. This helps the bacteria stick to and enter cells from the throat.
Streptococcus agalactiae (Group B Strep)
CylE protein is an enzyme that helps make a toxic pigment from Group B Strep. The pigment ornithine rhamnolipid harms red blood cells, platelets, and other immune cells [17], [18].
Pseudomonas aeruginosa
Adenylate cyclase disrupts immune responses and helps bacteria grow, making P. aeruginosa more harmful [19], [20].
Exoenzyme S messes up how macrophages move and change shape. This makes it harder for them to crawl around and swallow things [21]. It also stops epithelial cells from making DNA. This disrupts their structure and interferes with how they talk to each other [22].
Exotoxin A is the most harmful toxin from P. aeruginosa. It stops cells from making proteins, causing them to die [23].
Exoenzyme U interferes with cell shape and disrupts cellular barriers. It also causes tissue swelling [24].
Escherichia coli
Cytolethal distending toxin, subunits A, B, and C makes cells swell, damages DNA, and leads to their death. It’s also linked to causing long-lasting infections [25]. Since it destroys DNA, scientists think it may have a role in cancer promotion [26]. Escherichia coli bacteria that produce this toxin have been found in people with colon cancer [27]. This toxin has only been tested on animals, so we need more research to understand its effects on the gut. All subunits need to be present to have toxic effects.
Shiga toxin, subunits A and B stop cells from making proteins. Subunit A breaks the cell’s protein-making machinery (ribosomes). And subunit B helps subunit A enter cells by attaching to receptors on the cell’s surface [28]. Both subunits need to be present to have toxic effects.
Hemolysin A, alpha-hemolysin, can cause kidney inflammation and damage. It disrupts cell connections, triggers inflammation, and causes cell death [29], [30], [31]. E. coli strains with the gene for this toxin are more often linked to severe kidney infection than to bladder infection [32].
Enterobacteriaceae
Hemolysin E destroys red blood cells and other cells by making tiny holes in their membranes [33]. It helps bacteria become more dangerous by helping them stick to tissues, invade, and change how immune cells respond [34], [35].
Vibrio cholerae
Zonula occludens toxin makes the small intestine leakier. It does this by breaking the tight connections between their cells. This allows larger molecules to pass through the gut barrier [36].
Cholera enterotoxin, subunits A and B causes severe watery diarrhea in cholera disease [37]. Both parts A and B must be present for toxicity [38]. Not all types of V. cholerae make cholera toxin [39].
Helicobacter pylori
Vacuolating toxin disrupts cell function and can cause cells to die. It also limits immune cell growth. Which affects epithelial and stomach acid-producing cells [40], [41]. The toxin helps H. pylori survive in the stomach. Research links diseases like gastric cancer and peptic ulcers to this toxin [40].
Clostridioides difficile
Toxin A and toxin B damage cell structures, causing cell death. They also disrupt the gut lining and make it leakier. Both play a big role in causing the symptoms of C. difficile infection [42], [43].
Clostridium perfringens
Alpha toxin interacts with cell membranes, causing them to break apart [44]. It also weakens neutrophils, one immune cell that protects us from pathogens [45].
Probable enterotoxins A, B, C, and D may cause food poisoning and gut problems. They mess up how cells stick together in the gut, causing leaks and damage. Fluid loss can lead to symptoms like diarrhea, nausea, vomiting, and stomach cramps [46].
Beta 2 toxin causes holes in cell membranes [47]. Its toxic effects on humans are unclear [48]. In animals, it causes necrotic enteritis. An inflammatory condition that makes intestinal tissue die.


